Bond length is a fundamental concept in chemistry, reflecting the distance between the nuclei of two bonded atoms. This seemingly simple metric is influenced by a myriad of factors, each playing a crucial role in determining the stability and properties of molecules. Let’s delve into the key factors affecting bond lengths, illustrated with examples and summarized in tables for clarity.
1. Atomic Size
The size of the atoms involved in a bond is one of the most direct factors influencing bond length. Larger atoms have more distant electron clouds, resulting in longer bond lengths.
Example:
- H-H Bond (Hydrogen molecule): The bond length is about 0.74 Å (angstroms), which is relatively short due to the small size of hydrogen atoms.
- Cl-Cl Bond (Chlorine molecule): The bond length is about 1.99 Å, longer because chlorine atoms are larger.
| Molecule | Bond Length (Å) |
|---|---|
| H-H | 0.74 |
| Cl-Cl | 1.99 |
2. Bond Order
Bond order refers to the number of chemical bonds between a pair of atoms. A higher bond order generally means a shorter bond length due to increased electron density between the atoms, which pulls them closer together.
Example:
- C-C Single Bond: The bond length is about 1.54 Å.
- C=C Double Bond: The bond length decreases to approximately 1.34 Å.
- C≡C Triple Bond: The bond length further decreases to about 1.20 Å.
| Bond Type | Bond Length (Å) |
|---|---|
| C-C | 1.54 |
| C=C | 1.34 |
| C≡C | 1.20 |
3. Electronegativity
Electronegativity differences between bonded atoms can affect bond length. When there is a significant difference, the more electronegative atom pulls the bonding electrons closer, shortening the bond length.
Example:
- C-F Bond (in fluoromethane): Fluorine is highly electronegative, resulting in a bond length of about 1.39 Å.
- C-I Bond (in iodomethane): Iodine is less electronegative, leading to a longer bond length of about 2.14 Å.
| Molecule | Bond Length (Å) |
|---|---|
| C-F (CH3F) | 1.39 |
| C-I (CH3I) | 2.14 |
4. Hybridization
The type of hybridization of the bonding orbitals affects bond length. Bonds involving sp3 hybridized orbitals are generally longer than those involving sp2 or sp hybridized orbitals due to the different extents of orbital overlap.
Example:
- sp3 Hybridization (C-H in methane): Bond length is about 1.09 Å.
- sp2 Hybridization (C-H in ethene): Bond length is about 1.08 Å.
- sp Hybridization (C-H in ethyne): Bond length is about 1.06 Å.
| Molecule | Hybridization | Bond Length (Å) |
|---|---|---|
| C-H (CH4) | sp3 | 1.09 |
| C-H (C2H4) | sp2 | 1.08 |
| C-H (C2H2) | sp | 1.06 |
5. Resonance and Delocalization
Resonance structures can lead to bond lengths that are intermediate between single and double bonds due to delocalized electrons.
Example:
- Benzene (C-C bonds): All C-C bonds in benzene are of equal length (about 1.39 Å), which is between typical single and double bond lengths due to resonance.
| Molecule | Bond Length (Å) |
|---|---|
| Benzene (C-C) | 1.39 |
6. Formal Charge
Atoms bearing formal charges can alter bond lengths. Typically, bonds involving positively charged atoms are shorter due to increased electrostatic attraction, while those with negatively charged atoms are longer.
Example:
- Carbonate Ion (CO3^2-): The C-O bond length in CO3^2- is about 1.29 Å, shorter than a typical C-O single bond due to delocalization and formal charge distribution.
7. Lone Pair Repulsion
Lone pairs of electrons on adjacent atoms can repel each other, potentially increasing bond lengths.
Example:
- Hydrazine (N-N bond): The bond length is about 1.45 Å, longer than typical N-N single bonds due to lone pair repulsion.
8. Molecular Environment and Steric Factors
The surrounding atoms and overall molecular structure can influence bond lengths. Steric hindrance from bulky groups can lengthen bonds, while constrained environments can shorten them.
Example:
- Cyclopropane (C-C bonds): The C-C bond length in cyclopropane is about 1.51 Å, slightly shorter than in typical alkanes due to ring strain.
Summary Table of Key Factors
| Factor | Description | Example Molecule | Bond Length (Å) |
|---|---|---|---|
| Atomic Size | Larger atoms → longer bond lengths | H-H, Cl-Cl | 0.74, 1.99 |
| Bond Order | Higher bond order → shorter bond lengths | C-C, C=C, C≡C | 1.54, 1.34, 1.20 |
| Electronegativity | Higher electronegativity difference → shorter bond | CH3F, CH3I | 1.39, 2.14 |
| Hybridization | sp < sp2 < sp3 in bond length | CH4, C2H4, C2H2 | 1.09, 1.08, 1.06 |
| Resonance | Delocalization → intermediate bond lengths | Benzene | 1.39 |
| Formal Charge | Formal charges can shorten or lengthen bonds | CO3^2- | 1.29 |
| Lone Pair Repulsion | Lone pairs → longer bond lengths | Hydrazine | 1.45 |
| Steric Factors | Bulky groups or ring strain can affect bond lengths | Cyclopropane | 1.51 |
Understanding these factors provides a comprehensive picture of how various influences can alter bond lengths, thereby affecting the properties and reactivity of molecules. This knowledge is crucial for chemists in fields ranging from organic synthesis to material science, where precise control over molecular structure is essential.
Importance of Understanding the Factors that Affect Bond Lengths
Bond lengths are fundamental properties in chemistry that significantly influence the behavior, reactivity, and physical properties of molecules. Understanding the factors that affect bond lengths is crucial for several reasons, ranging from the design of new materials to the comprehension of biological processes. Here’s an in-depth look at why this understanding is important, with illustrative examples and applications.
1. Predicting Molecular Geometry and Stability
The geometry of a molecule, which includes bond lengths and angles, determines its overall shape and stability. By understanding the factors affecting bond lengths, chemists can predict and rationalize molecular geometries.
Example:
- Methane (CH4): Methane has tetrahedral geometry with C-H bond lengths of about 1.09 Å. Understanding hybridization (sp3 in this case) helps in predicting this geometry, which is crucial for understanding methane’s reactivity and physical properties.
2. Designing and Synthesizing New Molecules and Materials
In materials science and pharmaceuticals, the ability to design molecules with specific properties hinges on understanding bond lengths. Precise control over bond lengths can lead to materials with desirable mechanical, electrical, or optical properties.
Example:
- Carbon Nanotubes: These materials have exceptional strength and electrical properties. The C-C bond lengths in nanotubes are influenced by the hybridization and resonance within the carbon lattice. Understanding these factors allows scientists to manipulate the properties of nanotubes for various applications.
3. Interpreting Spectroscopic Data
Spectroscopic techniques such as X-ray crystallography, NMR, and IR spectroscopy rely on the accurate interpretation of bond lengths to determine molecular structures. Knowledge of factors affecting bond lengths aids in the correct interpretation of these data.
Example:
- X-ray Crystallography: This technique provides precise bond length measurements. Understanding how factors like electronegativity and hybridization affect bond lengths helps chemists confirm or revise the proposed structures of complex molecules.
4. Understanding Chemical Reactivity and Mechanisms
Chemical reactions often involve the breaking and forming of bonds. Knowing the factors that influence bond lengths helps chemists understand reaction mechanisms and predict reaction outcomes.
Example:
- Hydrogenation Reactions: In the hydrogenation of alkenes, the C=C double bond (1.34 Å) is converted to a C-C single bond (1.54 Å). Understanding the bond length changes and the factors influencing these lengths helps in optimizing reaction conditions.
5. Rationalizing Physical Properties
The physical properties of substances, such as boiling points, melting points, and solubility, are influenced by bond lengths and the resulting molecular structures.
Example:
- Boiling Points of Halomethanes: The boiling points of CH3F, CH3Cl, CH3Br, and CH3I increase as the C-X bond lengths increase (due to larger atomic sizes of halogens), affecting the van der Waals forces between molecules.
6. Enhancing Drug Design and Development
In medicinal chemistry, understanding bond lengths is vital for designing molecules that fit precisely into biological targets like enzymes or receptors. Accurate bond lengths ensure optimal binding and activity.
Example:
- Enzyme Inhibitors: Inhibitors designed to fit into the active site of an enzyme must have bond lengths that allow them to mimic the transition state of the enzyme’s natural substrate, leading to effective inhibition.
7. Environmental and Industrial Chemistry
Understanding bond lengths is crucial for developing environmentally friendly chemicals and processes. It aids in designing molecules that degrade into harmless substances or that efficiently catalyze reactions with minimal waste.
Example:
- Green Catalysts: Catalysts designed for industrial processes need precise bond lengths to facilitate specific reactions. Knowledge of how hybridization and electronic factors affect bond lengths helps in designing these catalysts.
Practical Applications in Education and Research
Educators and researchers benefit from a deep understanding of bond lengths in several ways:
- Curriculum Development: Teaching the factors affecting bond lengths equips students with the tools to understand complex chemical phenomena.
- Research: Accurate models and simulations in computational chemistry rely on precise bond length data, which is crucial for predicting molecular behavior and interactions.
Summary Table of Importance
| Area of Application | Importance | Example |
|---|---|---|
| Predicting Molecular Geometry | Determines shape and stability of molecules | Methane (CH4) |
| Material Design and Synthesis | Enables creation of materials with specific properties | Carbon Nanotubes |
| Spectroscopic Data Interpretation | Aids in determining accurate molecular structures | X-ray Crystallography |
| Chemical Reactivity and Mechanisms | Predicts reaction outcomes and optimizes conditions | Hydrogenation Reactions |
| Rationalizing Physical Properties | Explains variations in boiling/melting points | Halomethanes |
| Drug Design and Development | Ensures optimal binding and activity of pharmaceuticals | Enzyme Inhibitors |
| Environmental and Industrial Chemistry | Develops efficient and green chemical processes | Green Catalysts |
Conclusion
Understanding the factors that affect bond lengths is not just a theoretical exercise; it has practical implications across various fields of science and industry. From predicting the geometry and stability of molecules to designing new materials and drugs, this knowledge is fundamental to advancing technology and improving quality of life. As chemists and researchers continue to explore and manipulate bond lengths, the potential for innovation and discovery remains vast.


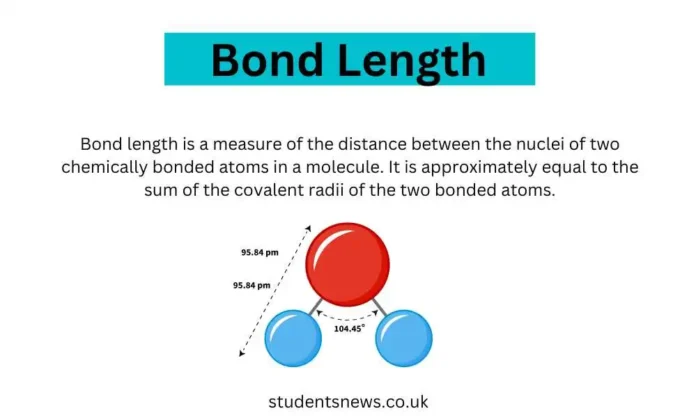
[…] in picometers (pm) or angstroms (Å), with 1 Å equal to 100 pm or 10^-10 meters. The exact length of a bond can be influenced by several factors, […]