Understanding Bond Length
Bond length is a measure of the distance between the nuclei of two bonded atoms in a molecule. It is a key parameter in understanding the structure and properties of molecules. Bond length varies depending on the type of atoms involved and the nature of the bond between them (single, double, triple, etc.).
Definition
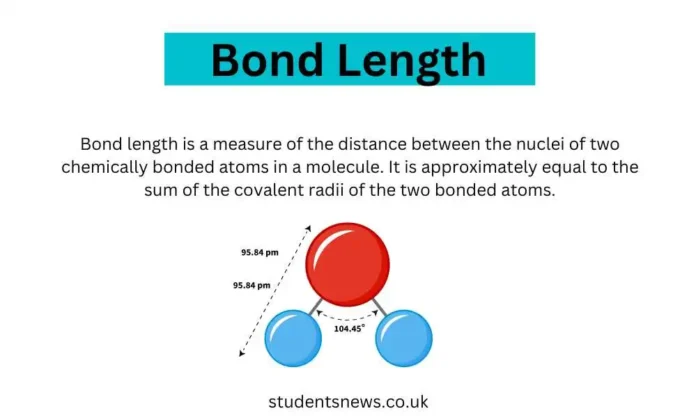
Bond length is the average distance between the nuclei of two bonded atoms in a molecule, indicating the equilibrium point where attractive and repulsive forces between atoms are balanced.
In a chemical bond, atoms are held together by the attractive forces between positively charged nuclei and the negatively charged electrons that are shared or transferred between atoms. The bond length represents the equilibrium distance at which these attractive and repulsive forces are balanced. At this distance, the energy of the system is minimized, making the molecular structure stable.
Bond lengths are typically measured in picometers (pm) or angstroms (Å), with 1 Å equal to 100 pm or 10^-10 meters. The exact length of a bond can be influenced by several factors, including:
- Bond Order: Generally, the more electrons that are shared between two atoms (higher bond order), the shorter the bond length. For example, a carbon-carbon single bond (C-C) is longer than a carbon-carbon double bond (C=C), which in turn is longer than a carbon-carbon triple bond (C≡C).
- Atomic Size: Larger atoms tend to form longer bonds because their outer electrons are farther from the nucleus, which weakens the bond between atoms.
- Electronegativity: Differences in electronegativity between bonded atoms can affect bond length. Polar bonds, where electrons are more attracted to one atom than the other, can have different bond lengths compared to nonpolar bonds between atoms of similar electronegativity.
- Hybridization: The hybridization of orbitals involved in bonding can also affect bond length. For example, in carbon compounds, sp hybridized orbitals form shorter bonds than sp2, which in turn form shorter bonds than sp3 hybridized orbitals.
Bond lengths are determined experimentally through techniques such as X-ray crystallography and spectroscopy. These measurements are crucial for the determination of molecular geometry, which helps in understanding the physical and chemical properties of substances, their reactivity, and interactions with other molecules.
Examples of Bond Lengths
Bond lengths can vary widely across different types of molecules and different types of chemical bonds. Here are some detailed examples to illustrate the range and factors influencing bond lengths:
Single Bonds Length Examples
- Hydrogen-Hydrogen (H-H): In a dihydrogen (H2) molecule, the bond length is approximately 74 picometers (pm). This bond is a simple covalent bond between two hydrogen atoms.
- Carbon-Hydrogen (C-H): In methane (CH4), the C-H bond length is about 109 pm. This bond length is typical for sp^3 hybridized carbon atoms bonded to hydrogen.
- Carbon-Carbon (C-C): In ethane (C2H6), the single bond between the two carbon atoms is about 154 pm, characteristic of sp^3-sp^3 hybridized carbon atoms.
Double Bonds Length Examples
- Carbon-Carbon (C=C): In ethene (C2H4), the double bond between the carbon atoms is shorter, at approximately 134 pm, reflecting the stronger bonding interaction compared to a single bond.
- Carbon-Oxygen (C=O): In formaldehyde (CH2O), the C=O bond length is around 120 pm. Double bonds involving oxygen are generally shorter due to oxygen’s higher electronegativity and the double bond’s partial π-character.
Triple Bonds Length Examples
- Carbon-Carbon (C≡C): In ethyne (acetylene, C2H2), the triple bond between the carbon atoms is even shorter, at about 120 pm, due to the increased electron sharing and stronger bonding interaction.
- Nitrogen-Nitrogen (N≡N): In molecular nitrogen (N2), the triple bond length is around 110 pm, one of the shortest and strongest bonds known, reflecting the strong triple bond between two nitrogen atoms.
Calculation of Bond Length
Calculating bond lengths in molecules involves understanding the principles of quantum chemistry and molecular orbital theory, which are used to describe the electronic structure of molecules. While exact calculations of bond lengths from first principles require complex computational methods, we can discuss some of the conceptual frameworks and simpler models that give insight into how bond lengths are determined and what factors influence them.
Quantum Chemistry and Molecular Orbital Theory
At the most fundamental level, the calculation of bond lengths involves solving the Schrödinger equation for the molecule, which describes how electrons behave under the influence of the nuclei. This is a complex problem because it requires accounting for the interactions between all the electrons and nuclei in a molecule. In practice, exact solutions are only possible for the simplest systems (like the hydrogen atom), and approximate methods must be used for more complex molecules.
Molecular Orbital (MO) Theory
Molecular orbital theory provides a way to understand bonding in molecules in terms of the combination of atomic orbitals to form molecular orbitals that are spread out over the entire molecule. Electrons in bonding molecular orbitals are in lower energy states than they would be in the separate atoms, leading to a stable bond. The energy difference between the bonding orbitals and the atomic orbitals gives the bond strength, and the distribution of electron density in these orbitals influences the bond length.
Valence Bond (VB) Theory and Hybridization
Valence bond theory describes bonds as the overlap of atomic orbitals from two atoms, which can hybridize to form new orbitals that are more effective in overlapping. The type of hybridization (sp, sp^2, sp^3, etc.) affects the bond angles and lengths. For example, sp hybridized carbons form shorter and stronger bonds than sp^3 hybridized carbons because the sp orbitals have more s-character, pulling the bonded atoms closer together.
Semi-Empirical and Empirical Methods
Given the complexity of accurately calculating bond lengths from first principles, chemists often use semi-empirical or empirical methods that rely on experimental data and simpler calculations. These methods can predict bond lengths based on known lengths in similar molecules, taking into account factors like bond order, atomic size, and electronegativity differences.
Example Calculation: Covalent Radius and Bond Length
One simplified approach to estimate bond length is to use the concept of covalent radii. The covalent radius of an atom is half the distance between two identical atoms bonded together in a molecule. By adding the covalent radii of two bonded atoms, one can estimate the bond length. For example:
- The covalent radius of a carbon atom (in a C-C single bond) is approximately 77 pm.
- Therefore, the estimated bond length of a C-C single bond would be 2 × 77 pm = 154 pm.
This method is a rough approximation and does not account for variations due to bond order, molecular environment, or other factors.
Computational Chemistry
For more accurate calculations, computational chemistry methods such as Hartree-Fock (HF) and post-Hartree-Fock (including configuration interaction (CI), Møller-Plesset perturbation theory (MP2), and coupled cluster theory (CC)) or density functional theory (DFT) are used. These methods involve calculating the electronic structure of molecules using numerical approaches.
- Hartree-Fock Method: Provides the wavefunction and energy of a quantum many-body system in a stationary state. HF calculations give a first approximation of molecular orbitals, electron distribution, and total energy, from which bond lengths can be inferred.
- Density Functional Theory (DFT): A computational quantum mechanical modeling method used to investigate the electronic structure (principally the ground state) of many-body systems, especially atoms, molecules, and the condensed phases. DFT allows for more accurate predictions of molecular properties, including bond lengths, by considering electron density rather than wavefunction.
Example of Computational Calculation
While a detailed computational calculation of bond length is beyond the scope of this text due to its complexity and the need for specialized software, the general process involves:
- Choosing a Computational Method: Selecting an appropriate level of theory (e.g., HF, DFT) and basis set (a set of functions used to describe the electron orbitals).
- Molecular Modeling: Creating a model of the molecule in a computational chemistry software.
- Optimization: Running a calculation that optimizes the geometry of the molecule, adjusting the positions of the atoms until the lowest possible energy configuration (the equilibrium geometry) is found.
- Analysis: Examining the output of the calculation to find the bond lengths within the optimized molecule.
These computational methods take into account the complex interplay of factors influencing bond lengths, including electron distribution, orbital hybridization, and electron-electron repulsions, providing accurate and reliable predictions that are in close agreement with experimental data.
In conclusion, while simple models and empirical data can provide estimates of bond lengths, accurate calculations require sophisticated computational methods that account for the quantum mechanical nature of electron interactions in molecules. These calculations are essential for understanding the structure, reactivity, and properties of chemical compounds.
Trend of Bond Length in Periodic Table
The trend of bond length within the periodic table is influenced by atomic size and electronegativity, which both vary in a predictable manner across periods (rows) and down groups (columns). Understanding these trends is crucial for predicting the properties of molecules and their reactivity. Here’s a general overview of how bond lengths tend to vary across the periodic table:
Across a Period (Left to Right)
- Decrease in Atomic Size: As you move from left to right across a period, atoms generally become smaller. This is because the number of protons in the nucleus increases, pulling the electron cloud closer and increasing the effective nuclear charge experienced by the electrons. Consequently, bonds formed between atoms later in a period tend to be shorter because the bonding electrons are closer to the nuclei.
- Increase in Electronegativity: Electronegativity also increases as you move from left to right across a period. This means that atoms towards the right of the period will more strongly attract the bonding electrons. However, the primary factor affecting bond length across a period is the decrease in atomic size, as the stronger pull on electrons due to increased electronegativity also contributes to the reduced atomic radius.
Down a Group (Top to Bottom)
- Increase in Atomic Size: Moving down a group in the periodic table, atoms increase in size. This is due to the addition of electron shells, which places the outermost electrons farther from the nucleus. As a result, bonds formed between atoms lower in a group are generally longer, given that the bonding electrons are further from the nuclei involved in the bond.
- Decrease in Electronegativity: Electronegativity tends to decrease down a group, meaning that atoms lower in the group are less effective at attracting bonding electrons. However, the dominant factor affecting bond length down a group is the increase in atomic size.
Specific Trends and Considerations
- Hydrogen Bonds: Hydrogen forms bonds with a relatively consistent length when bonded to nonmetals across periods 2 and 3, but the slight variations in bond lengths can be attributed to differences in electronegativity and atomic size of the other atom involved in the bond.
- Transition Metals: Bond lengths involving transition metals can vary significantly due to the involvement of d orbitals in bonding, which can lead to a wide range of bond lengths depending on the oxidation state of the metal and the nature of the ligands.
- Lanthanides and Actinides: For elements in the lanthanide and actinide series, the trend of increasing atomic size down the group is complicated by the lanthanide contraction, which is the gradual decrease in the size of lanthanide atoms from lanthanum to lutetium. This affects the bond lengths of compounds involving lanthanides.
- Covalent vs. Ionic Character: The bond length is also influenced by the type of bond (covalent or ionic) formed between atoms. Ionic bonds tend to be longer due to the larger size of ionized atoms compared to their neutral counterparts.
In summary, the trend of bond length in the periodic table reflects the underlying principles of atomic size and electronegativity. Bonds tend to become shorter across a period due to decreasing atomic size and increasing electronegativity, while bonds generally become longer down a group as atomic size increases. These trends are foundational for understanding molecular structure and reactivity in chemistry.
FAQs about Bond Length
What is bond length?
Bond length is the average distance between the nuclei of two bonded atoms in a molecule. It represents the equilibrium point where the energy of the system is minimized, balancing the attractive and repulsive forces between atoms.
How is bond length measured?
Bond length is typically measured using spectroscopic techniques, such as X-ray crystallography, infrared spectroscopy, and electron diffraction. These methods can accurately determine the distances between atoms in a molecule.
What factors influence bond length?
Several factors can influence bond length, including bond order (single, double, triple bonds), atomic size, electronegativity differences between the bonded atoms, hybridization of orbitals, and the presence of resonance structures.
Does bond length affect molecular properties?
Yes, bond length can significantly affect molecular properties, including reactivity, color, and physical state. Shorter bonds are generally stronger and more energy is required to break them, influencing the chemical behavior of the molecule.
Can bond length vary in a single molecule?
Yes, bond lengths can vary within a single molecule if it contains different types of bonds (e.g., single, double, triple) or if the molecular structure allows for resonance, leading to bond length equalization in some cases.
How does bond order affect bond length?
Higher bond orders (double, triple bonds) result in shorter bond lengths compared to single bonds. This is because more electrons are shared between the atoms, increasing the attractive force and pulling the atoms closer together.
Why are ionic bond lengths generally longer than covalent bond lengths?
Ionic bond lengths are longer because the bond involves the attraction between oppositely charged ions, which are typically larger than the neutral atoms forming covalent bonds. The size of the ions and the nature of ionic interactions contribute to the longer bond lengths.
Can bond length be predicted theoretically?
Yes, bond lengths can be predicted using theoretical methods and computational chemistry, including quantum mechanical calculations and molecular mechanics models. These methods use the principles of physics and chemistry to estimate the optimal distances between atoms in a molecule.
What is the significance of knowing bond lengths in chemistry?
Knowing bond lengths is crucial for understanding the structure and geometry of molecules, which in turn influences their chemical properties and reactivity. It helps chemists predict how molecules will interact in chemical reactions and design molecules with specific properties.
How does hybridization affect bond length?
Hybridization affects bond length by altering the electron density distribution around the bonded atoms. For example, sp hybridized orbitals have more s-character and are closer to the nucleus, leading to shorter bond lengths compared to sp^2 or sp^3 hybridized orbitals.
Are bond lengths constant under all conditions?
Bond lengths can vary slightly under different conditions, such as temperature, pressure, and the solvent in which the molecule is dissolved. These changes can affect the vibrational energy levels of the bond, leading to small variations in bond length.
Can bond lengths be used to identify unknown substances?
Yes, bond lengths can be characteristic of certain types of chemical bonds and molecular structures. By comparing measured bond lengths with known values, chemists can help identify unknown substances or confirm the presence of specific functional groups in a molecule.


[…] Bond Length: Find the length of the bond in meters (m). This is the distance between the nuclei of the two […]
[…] also, to a certain extent, influences the bond length […]
[…] Bond length is a fundamental concept in chemistry, reflecting the distance between the nuclei of two bonded atoms. This seemingly simple metric is influenced by a myriad of factors, each playing a crucial role in determining the stability and properties of molecules. Let’s delve into the key factors affecting bond lengths, illustrated with examples and summarized in tables for clarity. […]