What is an Ideal Gas?
An ideal gas is a theoretical concept in physics and chemistry used to model the behavior of gases under various conditions. The idea is based on several assumptions that simplify the behavior of real gases, allowing for easier mathematical treatment. These assumptions include:
- Molecules in Random Motion: The molecules of an ideal gas are in constant, random motion, colliding with each other and with the walls of the container.
- Negligible Molecular Volume: The volume of the individual gas molecules is so small compared to the volume of the container that it can be considered negligible. Essentially, the gas molecules are treated as point particles with no volume.
- No Intermolecular Forces: There are no attractive or repulsive forces between the gas molecules. The only interactions between molecules are elastic collisions, where kinetic energy is conserved.
- Elastic Collisions: Collisions between gas molecules and between molecules and the walls of the container are perfectly elastic, meaning there is no loss of kinetic energy during the collisions.
- Average Kinetic Energy Proportional to Temperature: The average kinetic energy of the gas molecules is directly proportional to the absolute temperature of the gas.
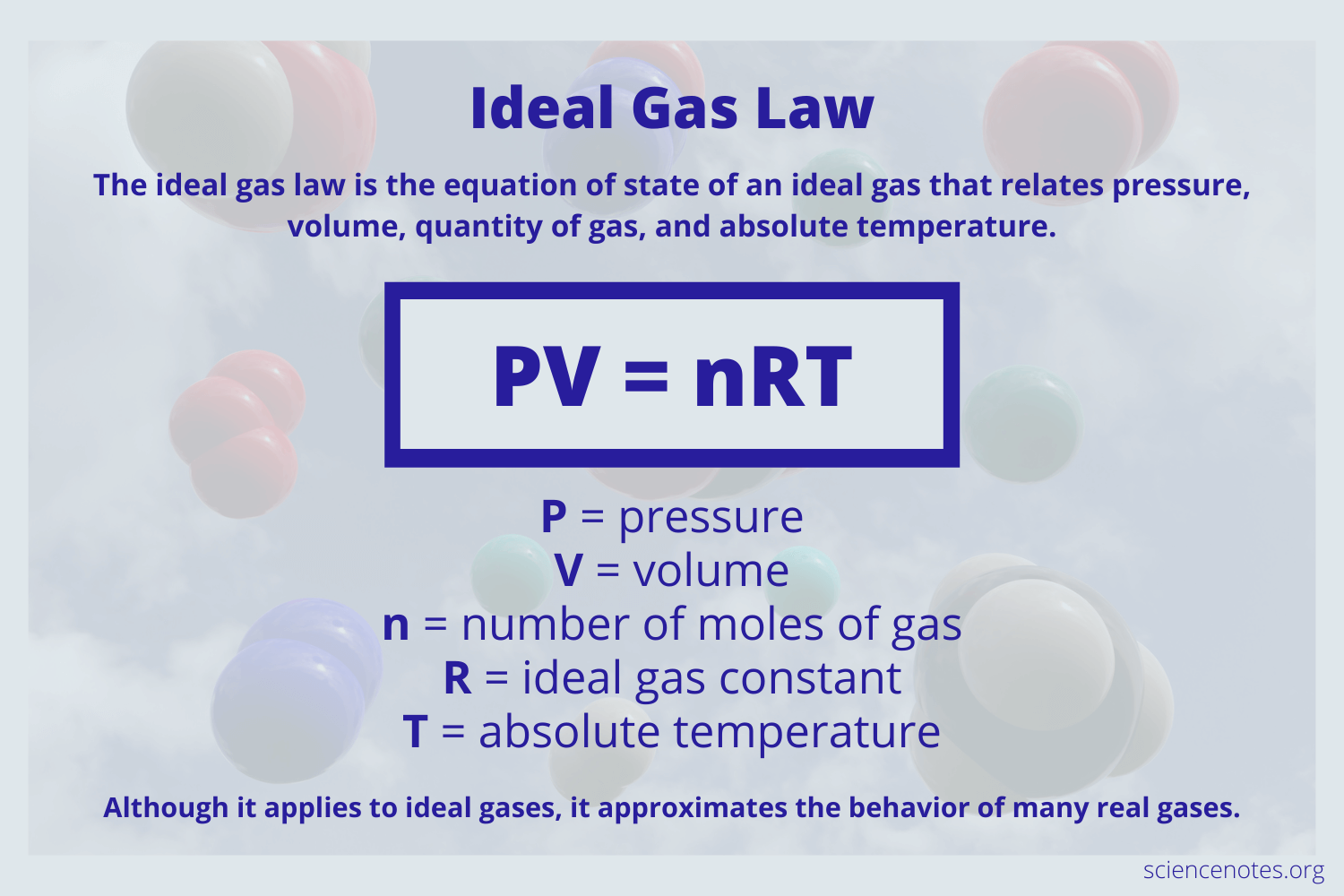
The behavior of an ideal gas is described by the Ideal Gas Law, which is an equation of state:
𝑃𝑉 = 𝑛𝑅𝑇
where:
𝑃 is the pressure of the gas,
𝑉 is the volume,
𝑛 is the number of moles of the gas,
𝑅 is the universal gas constant, and
𝑇 is the absolute temperature in Kelvin.
The ideal gas model is a good approximation for many gases under a wide range of conditions, especially at high temperatures and low pressures where the gas molecules are far apart and intermolecular forces are negligible. However, at very high pressures or low temperatures, real gases deviate from ideal behavior due to intermolecular forces and the finite volume of gas molecules. In such cases, more complex models like the Van der Waals equation are used to describe the gas behavior more accurately.
What is a Non-Ideal Gas?
A non-ideal gas, also known as a real gas, deviates from the ideal gas behavior described by the Ideal Gas Law due to the presence of intermolecular forces and the finite volume of gas molecules. The assumptions that define an ideal gas do not hold true for a non-ideal gas, particularly under conditions of high pressure and low temperature. The deviations arise because:
- Intermolecular Forces: In a real gas, molecules attract or repel each other. Attractive forces, such as van der Waals forces, become significant at high pressures or low temperatures, causing the gas to compress more than an ideal gas would. Repulsive forces come into play when molecules are very close together, as at very high pressures, causing the gas to occupy more volume than predicted by the Ideal Gas Law.
- Finite Molecular Volume: The gas molecules themselves occupy a finite, non-negligible volume. At high pressures, the volume occupied by the gas molecules becomes significant compared to the volume of the container, leading to deviations from ideal behavior.
To account for these deviations, various equations of state have been developed. One of the most commonly used is the Van der Waals equation, which modifies the Ideal Gas Law to include terms that account for intermolecular forces and molecular volume:
(P + a / Vm2)(Vm - b) = RT
where:
Pis the pressure,V mis the molar volume of the gas,Ris the universal gas constant,Tis the absolute temperature,ais a measure of the strength of the attractive forces between molecules,bis the volume occupied by one mole of gas molecules.
Other more sophisticated models and equations of state include:
- Redlich-Kwong Equation: An improvement over the Van der Waals equation, particularly at higher temperatures.
- Peng-Robinson Equation: Widely used in the petrochemical industry for its accuracy in predicting the behavior of real gases.
- Virial Equation: Expresses the relationship between pressure, volume, and temperature as a power series and includes coefficients that account for intermolecular forces.
In summary, non-ideal gases exhibit behavior that cannot be accurately described by the Ideal Gas Law, particularly under conditions of high pressure and low temperature. Adjustments and more complex models are necessary to account for intermolecular forces and the finite volume of gas molecules to predict the behavior of real gases more accurately.
So, what is the difference between an Ideal and Non-Ideal Gas?
here is a table comparing ideal and non-ideal gases:
| Feature | Ideal Gas | Non-Ideal Gas (Real Gas) |
|---|---|---|
| Intermolecular Forces | Assumes no intermolecular forces | Accounts for attractive and repulsive forces |
| Molecular Volume | Assumes negligible molecular volume | Accounts for the finite volume of gas molecules |
| Collisions | Collisions are perfectly elastic | Collisions may not be perfectly elastic |
| Equation of State | Follows the Ideal Gas Law: 𝑃𝑉=𝑛𝑅𝑇 | Described by more complex equations (e.g., Van der Waals) |
| Behavior | Linear and predictable relationship between P, V, T, n | Deviates from the Ideal Gas Law, especially at high pressures and low temperatures |
| Conditions for Validity | High temperature and low pressure | Significant at high pressures and low temperatures |
Can we say that Ideal gas is not Real?
An ideal gas is a theoretical construct used in physics and chemistry to simplify the study of gases. It assumes that gas molecules have no intermolecular forces and occupy no volume, which allows for the straightforward application of the Ideal Gas Law (𝑃𝑉=𝑛𝑅𝑇).
However, this idealization does not occur in nature. Real gases exhibit behavior influenced by intermolecular attractions and the finite volume of molecules, especially at high pressures and low temperatures.
Therefore, while the ideal gas model provides useful approximations under many conditions, it does not perfectly describe the behavior of real gases, which require more complex models like the Van der Waals equation.
Criteria to Judge whether a Gas is Ideal or Non-Ideal
To judge whether a gas behaves as an ideal or non-ideal gas, several criteria can be considered:
- Pressure:
- Low Pressure: Gases tend to behave more ideally at low pressures where intermolecular forces are minimal, and the volume occupied by the gas molecules is negligible compared to the container.
- High Pressure: At high pressures, gases deviate from ideal behavior due to the significant volume occupied by gas molecules and increased intermolecular forces.
- Temperature:
- High Temperature: Gases are more likely to behave ideally at high temperatures where the kinetic energy of molecules overcomes intermolecular attractions.
- Low Temperature: At low temperatures, gases show non-ideal behavior as intermolecular forces become more significant.
- Volume:
- Large Volume: When gases occupy a large volume, they tend to behave ideally since the effects of molecular volume and intermolecular forces are minimized.
- Small Volume: In smaller volumes, gases exhibit non-ideal behavior because the relative size of the molecules and intermolecular forces become more pronounced.
- Nature of the Gas:
- Simple Gases: Monatomic and diatomic gases (e.g., helium, hydrogen) often behave more ideally than complex molecules due to fewer intermolecular forces.
- Complex Gases: Larger, polyatomic gases with stronger intermolecular forces (e.g., water vapor, carbon dioxide) are more likely to exhibit non-ideal behavior.
- Intermolecular Forces:
- Weak Intermolecular Forces: Gases with weak intermolecular forces (e.g., noble gases) tend to behave ideally.
- Strong Intermolecular Forces: Gases with strong intermolecular forces (e.g., polar molecules) exhibit non-ideal behavior.
- Deviation from Ideal Gas Law:
- Minimal Deviation: Gases that closely follow the Ideal Gas Law (𝑃𝑉=𝑛𝑅𝑇) under a wide range of conditions can be considered ideal.
- Significant Deviation: Gases that show significant deviations from the Ideal Gas Law, particularly under varying pressures and temperatures, are considered non-ideal.
By evaluating these criteria, one can determine whether a gas is likely to behave as an ideal gas or a non-ideal gas under specific conditions.
Conclusion
In conclusion, the ideal gas is a theoretical model assuming no intermolecular forces and negligible molecular volume, accurately described by the Ideal Gas Law (𝑃𝑉=𝑛𝑅𝑇).
This model provides useful approximations under high temperature and low pressure conditions but does not exist in reality. Real gases, which exhibit intermolecular forces and finite molecular volume, deviate from ideal behavior, especially at high pressures and low temperatures.
These deviations necessitate more complex equations, such as the Van der Waals equation, to accurately describe their behavior. Thus, while ideal gas laws are valuable for approximations, real gas behavior requires consideration of non-ideal factors.